
Palmitoylethanolamide For Occasional and Minor Pain Support*
400 mg
90 Vegetarian Capsules ( SKU: 9331U )
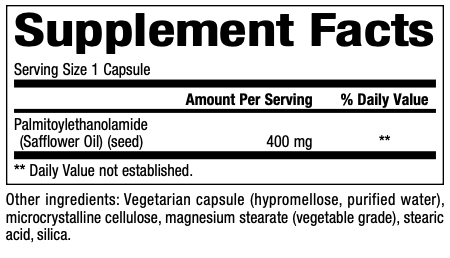
Supplement Facts:
Dosage:
Suggested Usage: 1 capsule 1–3 times per day or as directed by a health care professional.
Warnings:
Consult your health care professional prior to use if you are pregnant, trying to become pregnant, breastfeeding, taking medication, have a medical condition, or anticipate surgery. Keep out of reach of children.
Allergens:
Contains no artificial colors, preservatives, or sweeteners; no dairy, sugar, wheat, gluten, yeast, soy, sesame, corn, egg, fish, shellfish, animal products, salt, tree nuts, or GMOs. Suitable for vegetarians/vegans. Sealed for your protection. Do not use if seal is broken. For freshness, store in a cool, dry place.
