
5 mg Time Release
5 mg
60 Tablets ( SKU: 9290U )
Supplement Facts:
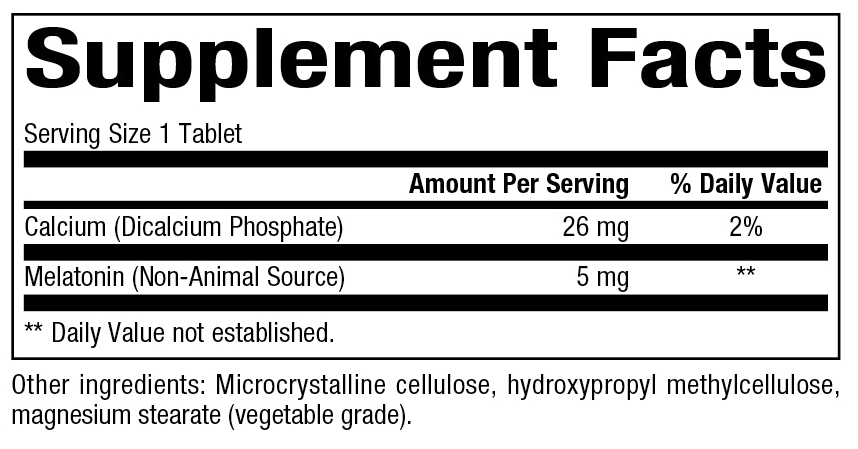
Dosage:
Suggested Usage: 1 tablet per day at bedtime or as directed by a health care professional.
Allergens:
Contains no artificial colors, preservatives, or sweeteners; no dairy, starch, sugar, wheat, gluten, yeast, soy, corn, egg, fish, shellfish, animal products, salt, tree nuts, or GMOs. Suitable for vegetarians/vegans.
