
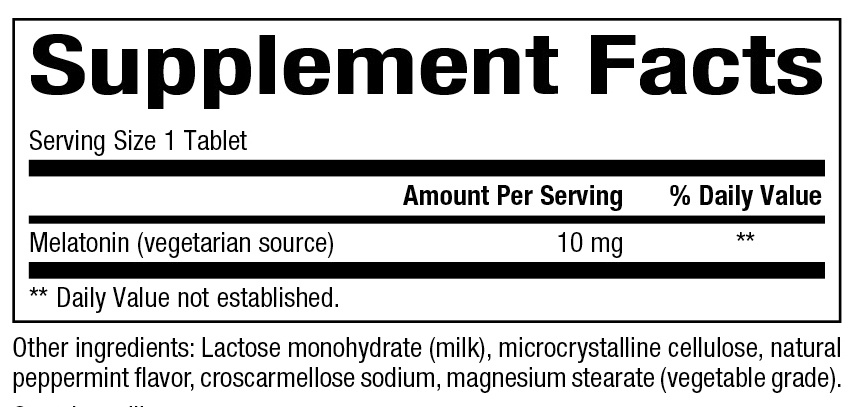
10 mg
10 mg
180 Quick Dissolve Tablets ( SKU: 9286U )
Supplement Facts:
Dosage:
Suggested Usage: Chew or dissolve 1 tablet at bedtime in the mouth before swallowing or as directed by a health care professional. For use beyond 4 weeks, consult a health care professional.
Warnings:
Consult a health care professional prior to use if you have a hormonal disorder, diabetes, liver or kidney disease, cerebral palsy, seizure disorders, migraine, depression and/or hypertension, or if you are taking blood pressure or sedative/hypnotic medications. Do not use if you are taking immunosuppressive drugs and/or if you are pregnant or breastfeeding. Do not drive or use machinery for 5 hours after taking melatonin. If symptoms persist continuously for more than 4 weeks (chronic insomnia), consult your health care professional. Keep out of reach of children.
Allergens:
Contains no artificial colors, preservatives, or sweeteners; no starch, sugar, wheat, gluten, yeast, soy, egg, fish, shellfish, salt, tree nuts, or GMOs. Suitable for vegetarians.
