
Maintains Proper Muscle Function* Supports Metabolism*
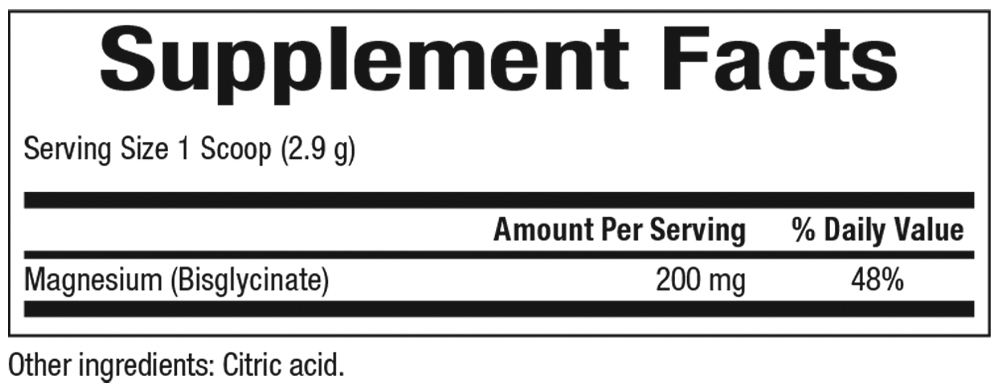
200 mg
5.1 oz ( SKU: 9500U )
Supplement Facts:
Dosage:
Suggested Usage: Mix 1 scoop (approx. 2.9 g) into water or juice once per day or as directed by a health care professional.
Warnings:
Consult your health care professional prior to use if you are pregnant, trying to become pregnant, breastfeeding, taking medication, have a medical condition, or anticipate surgery. Keep out of reach of children.
Allergens:
Contains no artificial colors, preservatives, or sweeteners; no dairy, starch, sugar, wheat, gluten, yeast, soy, corn, egg, fish, shellfish, animal products, salt, tree nuts, or GMOs. Suitable for vegetarians/vegans.
